Introduction into the |
Science and Fun Spectroscopic Tools |
Step: 128
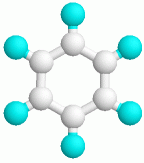
The reference axis of the benzene is C6 , because for it first of the criteria indicated in step 124 applies that this axis possesses the highest tenacity. Since the molecule is neither linear nor cubic, we are, like also with the NH3 , in the part of the algorithm, where it between S 2n , which C and D- point groups apply to decide.
2. Further symmetry elements:
According to the algorithm the following questions result: Is S 12 kolinear to C6 ? No.
Are six C2 perpendicular in one level to the reference axis? Yes.
Is sh available to the reference axis? Yes.
Check these questions conscientiously!
Thus the group of points of the benzene results too...?